How trifluoroacetic acid from breakdown of some F-gases occurs in nature and is completely different to most PFAS
The UNEP Environmental Effects Assessment Panel (EEAP) in its latest 2021 scientific update includes a comprehensive summary for Trifluoroacetic acid (TFA) and points out that most PFAS have different properties from TFA. Some HFCs and some HFOs breakdown produce TFA in the atmosphere. Here are some simple facts and charts that explain why. The EEAP comprehensive summary was reported in the EFCTC March newsletter.
Definitions: TFA stands for trifluoroacetic acid and its salts, the fluorinated equivalent of acetic acid found in vinegar. TFA is a natural substance with over 200 million tonnes in the oceans and hydrosphere. Per- and polyfluoroalkyl substances (“PFAS”) covers a broad range of [anthropogenic] substances that according to currently accepted terminology contain at least one perfluoralkyl group.
Why are some PFASs a particular concern? The initial focus was on long chain perfluoroalkyl acids (PFAAs) and their precursors, in particular perfluorooctanoic acid (PFOA) and its derivatives and perfluorooctane sulphonic acid (PFOS) and its derivatives. Long-chain PFAAs are typically identified as highly persistent (P), bioaccumulative (B), and toxic (T), are ubiquitously distributed in the environment and recognised as global contaminants of high concern. In contrast TFA does not bioconcentrate in aquatic organisms and does not biomagnify in the food chain and TFA salts are of low acute toxicity to mammals under conditions relevant to environmental exposure.
Why are the properties so different between TFA and long chain PFAAs?
Octanol water partition coefficient (KOW) is a key parameter in studies of environmental fate of chemical substances. It is usually expressed as log KOW with values typically between −3 (very hydrophilic) and +10 (extremely hydrophobic). Log KOW is relevant to partitioning between water and sediments, soil adsorption, biological uptake, lipophilic storage and biomagnification.
• High Log KOW greater tendency to partition to organic phase
• Low log KOW greater tendency to partition to aqueous phase
• For these acids water solubility and Log KOW follow the same trends
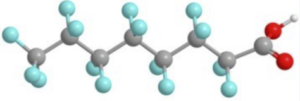
| PFOA has a very hydrophobic long chain perfluorinated group and is a strong acid due to the perfluorinated group making it an effective surfactant with low water solubility. It has a high water octanol partition coefficient KOW |
|
| TFA is not hydrophobic, is a strong acid and is highly soluble in water. It has a low water octanol partition coefficient KOW, about 2000 times lower than PFOA. In water it will be present as the perfluorocarboxylate CF3COO- | 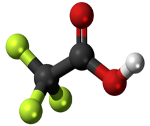 |
Comparing Perfluorocarboxylic acids and the acetates for water solubility and Log KOW
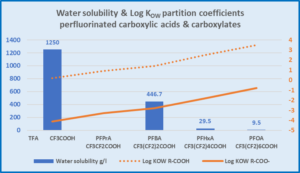 |
TFA highly water soluble, TFA acetate very low KOW As chain length increases water solubility decreases and KOW increases PFOA has low water solubility and a high KOW about 2000 times higher than TFA. |
Comparing Perfluorocarboxylic acids and carboxylic acids
| Acetic acid has very different properties to octanoic acid, which is a medium chain length fatty acid. TFA trifluoroacetic acid has very different properties to PFOA, perfluoro-octanoic acid. |
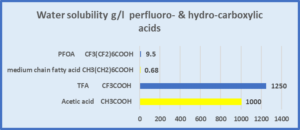 |
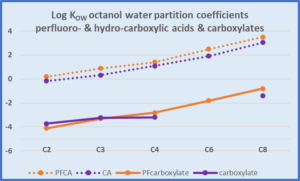 |
Trifluoroacetic acid & acetic acid are both: • very hydrophilic • high water solubility • low log KOW for the acids • very low log KOW for the carboxylatesTFA is present only as the carboxylate CF3COO- as it is a strong acid. |